Medical device manufacturing is one of the world’s most crucial and life-saving industries. It deals with multiple components, such as syringes, hypodermic needles, health-checking tools, implanted defibrillators, female diagnostics, and cardiac monitoring equipment.
Medical devices include various products for diagnosing, preventing, relieving, or treating disease and injury. More than 5 lakh types of medical devices are available, covering everything from wheelchairs and glasses to pacemakers and state-of-the-art surgical equipment.
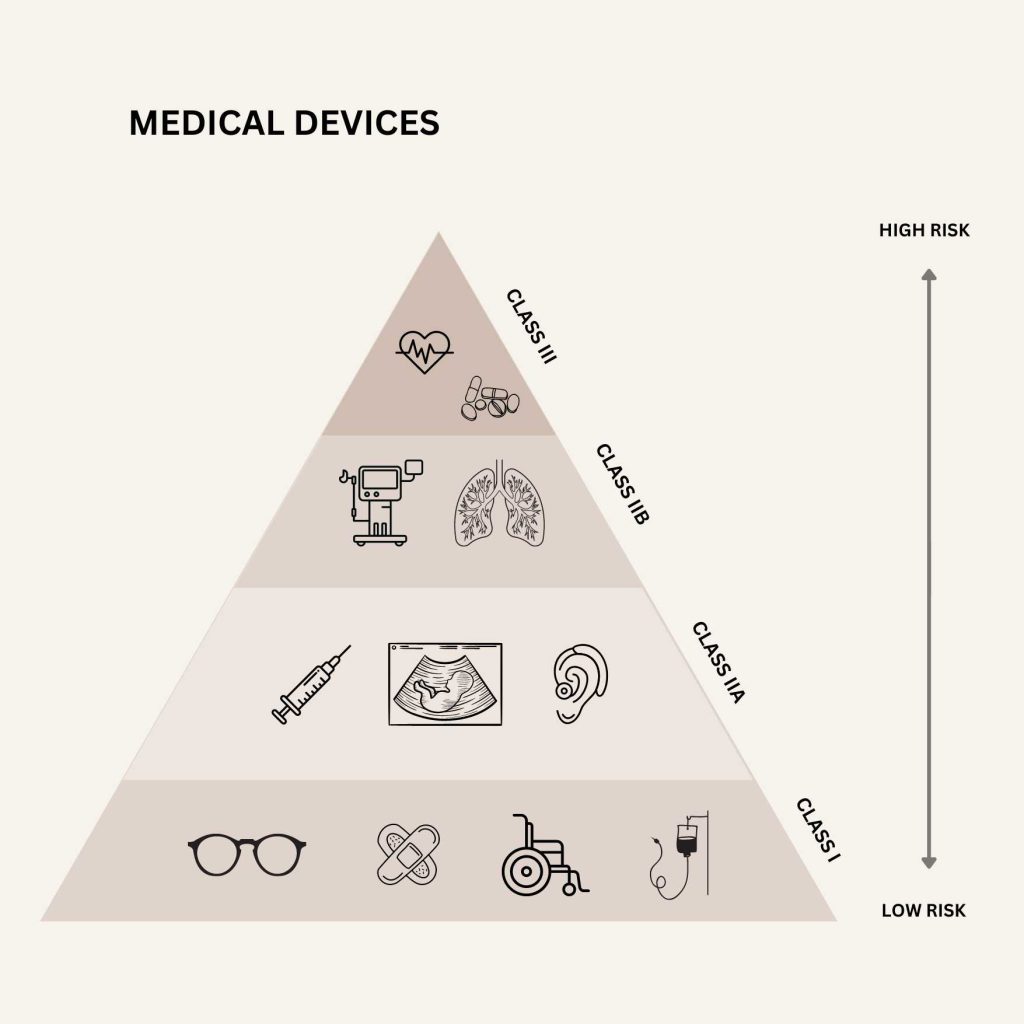
Medical devices are classified into four risk categories: Classes I, IIa, IIb, and III. Class I devices are the least risky, and Class III is the most difficult.
List of Medical Devices in Manufacturing Businesses:
- Blood pressure monitor
- Thermometer
- Electrocardiogram (ECG or EKG) machine
- X-ray machine
- MRI scanner
- Ultrasound machine
- Defibrillator
- Pacemaker
- Insulin pump
- Ventilator
- Nebulizer
- Hemodialysis machine
- Glucometer
- Otoscope
- Ophthalmoscope
- Surgical microscope
- Laser scalpel
- Endoscope
- Heart rate monitor (electronic)
- Pulse oximeter
As the population increases and health concerns continue growing, medical device manufacturers seek new technological solutions to address these challenges.
What are the Challenges in Medical Device Manufacturing?
Although technology and trends in the medical equipment industry change constantly, manufacturers should ensure that their devices meet high-quality standards. Here are some of the challenges faced by this industry:
Regulatory Challenges: The medical device industry is heavily regulated to ensure patient safety and product effectiveness. Delays can occur if the device fails to meet regulatory requirements, leading to a lengthy approval process. Changes in regulations or unexpected regulatory hurdles can also cause setbacks.
Design and Development Issues: If the initial design and development of the medical device are adequately planned and executed, it can lead to setbacks. Design flaws, inadequate testing, or changes in requirements can result in redesigns and delays.
Supply Chain Disruptions: Medical devices often require specialized components or materials. Any disruption in the supply chain, such as shortages of essential parts, can lead to production delays.
Quality Control Problems: Ensuring the quality and reliability of medical devices is crucial. If quality control processes are not adequately implemented, it can lead to manufacturing defects, product recalls, and production delays.
Manufacturing Process Complications: Complex manufacturing processes can lead to unforeseen complications. Issues with equipment, process validation, or scaling up production can cause delays.
Testing and Validation Delays: Thorough testing and validation are necessary to ensure the safety and efficacy of medical devices. Delays in testing, problems with test results, or failures to meet performance criteria can all contribute to setbacks.
Documentation and Record-Keeping: Proper documentation and record-keeping are essential in the medical device industry to demonstrate compliance with regulations. Inadequate documentation or errors in records can lead to delays during regulatory inspections.
Human Error: Mistakes made by personnel during various manufacturing stages, such as assembly, packaging, and labeling, can lead to defects or non-compliance issues, resulting in setbacks.
Supplier Issues: Suppliers failing to meet quality standards or encountering production issues can impact the overall manufacturing timeline.
Unforeseen External Factors: External factors like natural disasters, geopolitical events, or pandemics (as demonstrated by COVID-19) can disrupt supply chains, transportation, and manufacturing operations, causing significant delays.
Change Management Challenges: Introducing changes to a manufacturing process or device design can lead to complications if not managed effectively. Changes need to be carefully evaluated and validated to prevent setbacks.
Testing and Clinical Trials: Clinical trials and testing on human subjects are required for more complex medical devices. Delays in recruitment, adverse events, or unexpected trial results can extend development timelines.
What is the solution for setbacks in medical device manufacturing
ERP software has become essential for modern businesses. In every industry, along with medical device manufacturing, to name a few, almost every company invests in ERP systems that can integrate every part of the business and streamline complex manufacturing processes.
Acumatica cloud ERP software offers business management tools that optimize order planning and fulfillment, handle underutilized inventory and stock-outs, ensure better quality standards, and manage BOMs (bills of materials) and every manufacturing unit area.
Acumatica’s ERP solution for medical device manufacturers provides real-time visibility into the supply chain. It checks the quality of every medical equipment received, procured, shipped, and stored in final assembly. Please get in touch with us for more information about how ERP can help you execute your goals and reach your objectives in the medical device manufacturing industry. Contact us for a customized ERP.

Vijay comes with a vast experience in ERP and enterprise solutions space with about 20 years of experience in various packaged application like Acumatica, SAP, Orion, Salesforce.com, SugarCRM and, SalesLogix.
















